Which of these molecules is the most polar? a. CH3Cl b. C2H6 c. CH3CHO d. CO2 e. all are nonpolar | Homework.Study.com

MAYHAN Ch. 7-8 Review Sheet. 1. What are the properties of IONIC substances? These substances: -Solid Hard and brittle (like salt) at room temp -Conduct. - ppt download

Periodic Table Groups 1. Color-code the following groups on your periodic table: -alkali group -alkaline group - transitional group -halogen group -noble. - ppt download

PPT - Bonds can be classified as being either polar or non-polar . PowerPoint Presentation - ID:9663902

Which type of intermolecular force ("interparticle force") is the most important in CI4(s)? 1. Ionic bonds 2. Dipole-dipole forces 3. Hydrogen bonds 4. Ion-dipole forces 5. London Dispersion | Homework.Study.com

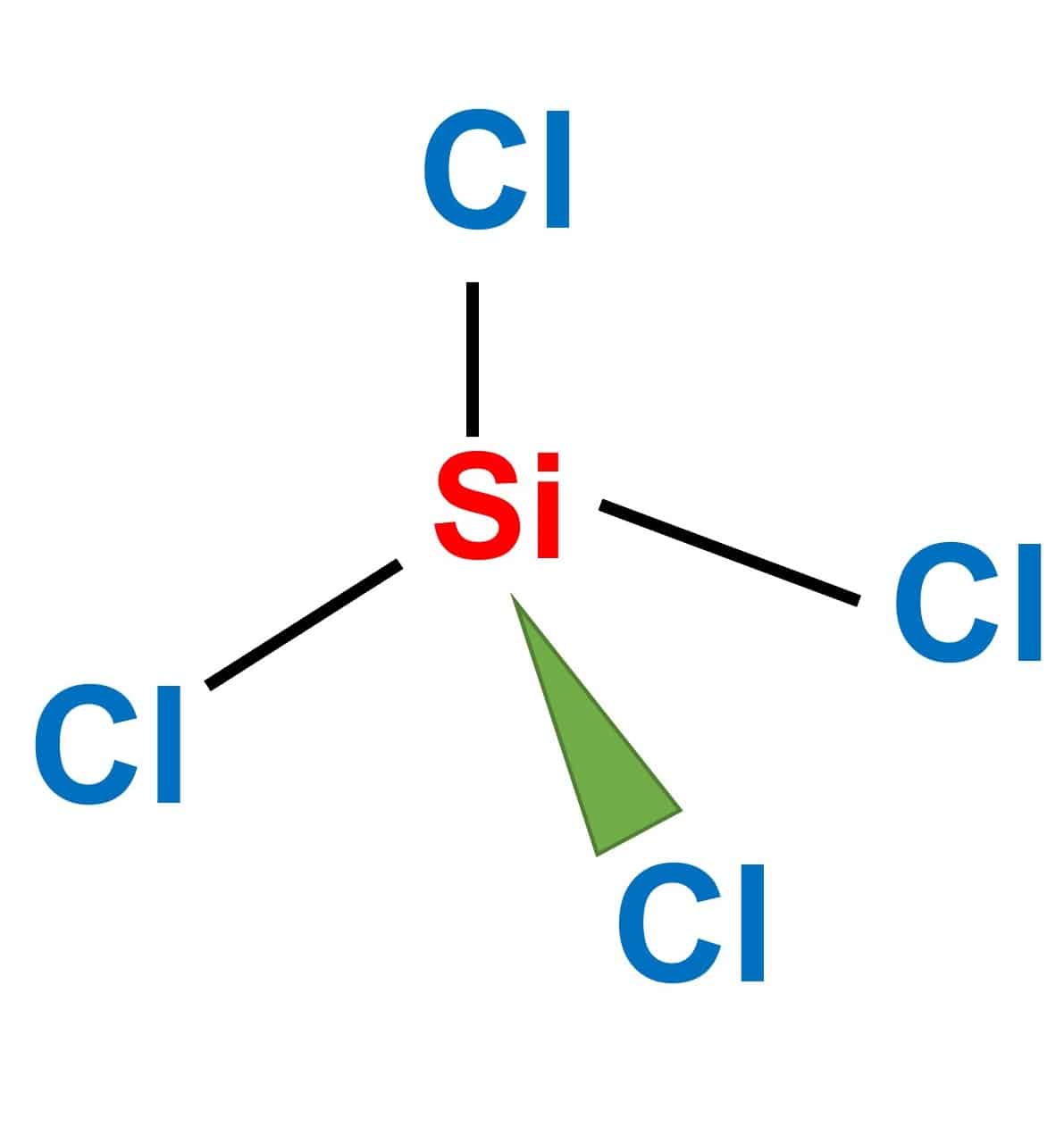
Which of the following molecules could exist in both polar and nonpolar forms, depending on the arrangement of the halogen atoms? a. XeFCl b. XeCl2F2 c. SiCl2F2 | Homework.Study.com

SOLVED: Choose the compound below that contains at least one polar covalent bond, but is nonpolar HCN IF3 SeF4 CBr4 Both B and C are nonpolar and contain a polar covalent bond.

SOLVED: Choose the compound below that contains at least one polar covalent bond, but is nonpolar: GeH2Cl2, Si2, AsF5, CBr2Cl2. All of these are nonpolar and contain a polar covalent bond.

Chemical Bonding: Objectives: Illustrate chemical bonding using Lewis Dot structures. Establish chemical formula and name of ionic compounds. - ppt download